iodine has how many valence electrons|Valence Electrons Chart for All Elements : Bacolod The elements in the periodic table are arranged according to their atomic number. Since rhodium is the 53rd element of the periodic table, the atomic number of iodine is 53. We must always remember that the atomic number and the number of . Tingnan ang higit pa Children find it particularly difficult to recognise if zero is odd or even. "A survey of primary school children in the 1990s showed that about 50% thought zero is even, about 20% thought it was .
PH0 · What is the number of core and valence electrons for I?
PH1 · Valences of the Chemical Elements
PH2 · Valence Electrons Chart for All Elements
PH3 · Khan Academy
PH4 · Iodine Valence Electrons (And How to Find them?)
PH5 · Iodine Valence Electrons
PH6 · How to Find the Valence Electrons for Iodine (I)
PH7 · How many valence electrons does Iodine have?
PH8 · How Many Valence Electrons Does Iodine (I) Have?
PH9 · 3.1: Valence Electrons
Explore an unconventional spin on craft cocktailing and wine at The Rickey Cocktail Lounge located at the Dream Midtown hotel in NYC. Skip to Content. Reservations. Reservations. . The bar’s furnishings and subtle design details are all one of a kind. Handpicked lamps, created by the self-proclaimed Steampunk Illusionist Terry Peikin, are .
iodine has how many valence electrons*******The third step is to diagnose the valence shell. The last shell after the electron configuration is called the valence shell. The total . Tingnan ang higit paThe elements in the periodic table are arranged according to their atomic number. Since rhodium is the 53rd element of the periodic table, the atomic number of iodine is 53. We must always remember that the atomic number and the number of . Tingnan ang higit paStep 2 is very important. In this step, the electrons of iodine have to be arranged. We know that iodine atoms have a total of fifty-three . Tingnan ang higit pa Mar 23, 2023 iodine has how many valence electrons Valence Electrons Chart for All Elements May 19, 2024
There are two ways to find the number of valence electrons in Iodine (I). The first is to use the Periodic Table to figure out how many electrons Iodine has in its .
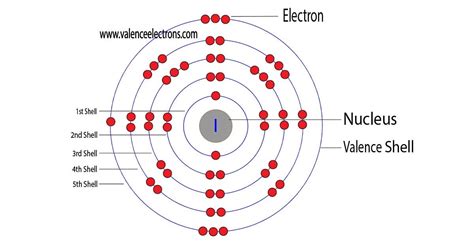
How Many Valence Electrons Does Iodine (I) Have?
How many valence electrons does Iodine have? - BYJU'SHow Many Valence Electrons Does Iodine (I) Have?How many valence electrons does Iodine have? - BYJU'S
Valency of Iodine. The valency of Iodine remains -1 for most of its states. The chemical element has 7 electrons in the outer shell of energy. Hence Iodine gains 1 .The valence electrons are the sum of the electrons in the outermost shell, that is two 5 s electrons and five 5 p electrons which gives a total of seven valence electrons. .
An iodine atom has seven valence electrons. Do you think it will lose seven electrons or gain one electron to obtain an octet in its outermost electron shell? . If you're seeing this message, it means we're having trouble loading external resources on our website. If you're behind a web filter, please make sure that the domains *.kastatic.org and . Iodine has 7 valence electrons because there are 7 electrons present in the outermost shell of the Iodine (I) atom. Now let’s see how you can easily find the . Iodine has 7 valence electrons and 46 core electrons for a total of 53, the atomic number. Explanation: Iodine is in the group 17 elements, known as halogens, .A neutron is one of the subatomic particles that make up matter. In the universe, neutrons are abundant, making up more than half of all visible matter.It has no electric charge and a rest mass equal to 1.67493 × 10−27 kg—marginally greater than that of the proton but nearly 1839 times greater than that of the electron.The neutron has a mean square .
iodine has how many valence electrons This table of element valences includes the maximum valence and most common valence values in chemistry. . You may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that can be derived by . Iodine-1, +1, (+3), (+4), +5, +7: 54: Xenon: 0: 55: Cesium +1: 56: . However, the most common valency of Iodine is -1, +1, +3, +5, or +7. This is because Iodine has 7 valence electrons, and it can either gain or lose electrons to complete its octet (or duplet, in the case of +1 valency) in chemical reactions. For example, in the compound sodium iodide (NaI), Iodine has a valency of -1, meaning it has .b) How many unpaired electrons does iodine have? To find the answer we refer to part a) and look at the valence electrons. We see that iodine has 5 electrons in the p orbitals. We know that the full p orbitals will add up to 6. Using the Hund's rule and Pauli exclusion principals we can make a diagram like the following: The answer is one. 3. How many valence electrons are in one atom of each element? sulfur; helium; potassium; aluminum; Solution. Sulfur (S) is located in Group VIA (Group 16), so it has 6 valence electrons. Helium (He) is located in Group VIIIA (Group 18). However, one atom only has two electrons, so it could never have more than 2 valence electrons. There are two ways to find the number of valence electrons in Iodine (I). The first is to use the Periodic Table to figure out how many electrons Iodine has .Anything that influences the valence electrons will affect the chemistry of the element.which one of the following factors does not the valence shell? (a) valence principal quantum number (n) (b) Nuclear charge (Z) (c) Nuclear mass (d) Number of core electrons.
Iodine has seven valence electrons. Valence electrons of an atom are located in the outermost shell of the atom and participate in bonding. If an.
If you're seeing this message, it means we're having trouble loading external resources on our website. If you're behind a web filter, please make sure that the domains *.kastatic.org and .
Steps for Writing Lewis Structures. Example \(\PageIndex{2}\) 1. Determine the total number of valence electrons in the molecule or ion. Each hydrogen atom (group 1) has one valence electron, carbon (group 14) has 4 valence electrons, and oxygen (group 16) has 6 valence electrons, for a total of [(2)(1) + 4 + 6] = 12 valence electrons. 2. Figure \(\PageIndex{1}\): Electrons are much smaller than protons or neutrons. If an electron was the mass of a penny, a proton or a neutron would have the mass of a large bowling ball! . and each has a 1− charge and a mass number of 127. Determine the numbers of protons, neutrons, and electrons in one of these iodine anions.In the IBr4- ion, how many electron groups surround the central iodine atom? How many valence electrons are in a phosphorus atom? How many valence electrons does the atom radium (Z = 88) have? How many valence electrons does the element N have? How many valence electrons are in the outermost shell of all halogens? a. 1 b. 4 c. 7 d. 8
Iodine is a group VIIA element in the periodic table and contains seven electrons in its last shell. Now, we know how many electrons are there in valence shells of iodine atoms. valence electrons given by iodine atoms = 7 * 2 = 14; Total valence electrons = 14; Total valence electrons pairs Iodine Number of Valence Electrons. Iodine has seven Valence Electrons. What is The Electron Configuration of Iodine. There are 53 electrons in iodine that occupy the respective orbitals as given below; 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰ 4p⁶ 5s² 4d¹⁰ 5p⁵ .the ion ICl4- has-----valence electrons.a. 8b. 35c. 34d. 28e. 36 Your solution’s ready to go! Our expert help has broken down your problem into an easy-to-learn solution you can count on.
Step 4: Find Valence Electrons. The total number of electrons present in the valence shell of an atom is called valence electrons, and there is only one electron present in the valence shell of xenon (5s²5p⁶). Thus, xenon has eight valence electrons. Valency of Xenon (Xe)

How many valence electrons does hydrogen ion(H +) have? Elements that have 1, 2, or 3 electrons in their last orbit can easily turn into positive ions by donating electrons. Those atoms that donate electrons and turn into positive ions are called cations. The first step is to count all the valence electrons of each molecule. In the case of IF5, The Iodine atom has 7 valence electrons. F also has 7 valence electrons. But since there are 5 atoms of F, we multiply 7×5= 35 valence electrons. Adding both we get 35+7= 42. Hence, a total number of valence electrons of IF5= 42. 2. Determining .
Benefits Effects Weight loss By Lou Mudge. . "The stomach vacuum exercise involves contracting and holding in your stomach muscles while breathing deeply. The goal is to strengthen the transverse abdominis muscle, the deepest layer of the abdominal muscles, creating a slimming effect on the waistline." .This is the current news about hms ritm.gov.ph/hms/register|ARSP is now accepting applicants for ARSP Accreditation 2022
iodine has how many valence electrons|Valence Electrons Chart for All Elements